CDD: smart00248.5,
ANK
PFAM: PF00023
InterPro: IPR002110
SMART: SM0248

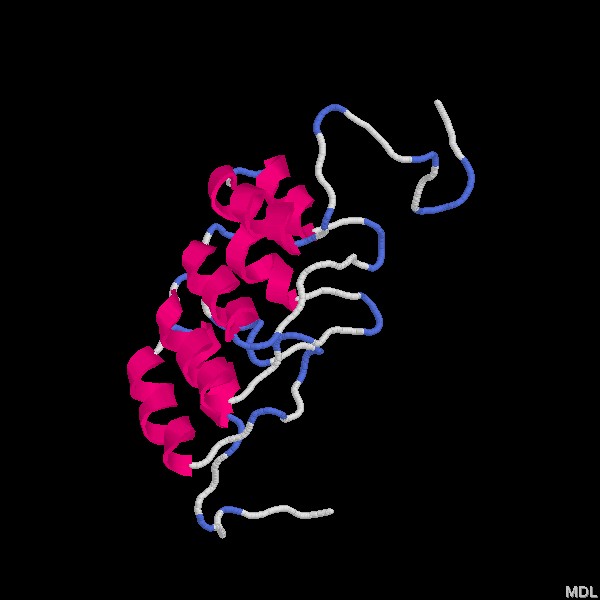
Homo sapiens ankyrin repeat domain of Bcl-3
Authors: F. Michel, M. Soler-Lopez, C. Petosa,
P. Cramer, U. Siebenlist, C. W. Mueller
pdb file: 1K1A
jpeg generated from pdb file 1K1A
using Chime
Description
Ankyrin repeat domains consist of repeated sequences approximately 33 amino acids long. These sequences have been known to occur in several consecutive copies as shown above. Ankyrin repeats are characterized as L-shaped in structure, consisting of a b-hairpin and two a-helices.
Ankyrin repeat regions are known to function as interactive domains between proteins. They have been described in a large number of functionally diverse proteins found mainly in eukaryotes. Ankyrin repeats are incorporated in several diverse families of proteins including ankyrins, which link integral membrane proteins to the spectrin-actin cytoskeleton and have involvement in cell motility, activation, proliferation, and contact.
A few known examples have also been isolated in prokaryotes
and viruses and are suspected of originating as the result of horizontal
gene transfers.
Examples of proteins containing the ANK domain
ANK1
Also known as ankyrinR. This membrane protein attaches
cytoskeletal components to the plasma membrane. This protein was
first isolated in human erythrocytes and later found in the brain and muscle
tissue.
PubMed: 9235914
LocusLink: 286
ANK3
Also known as ankyrinG. This protein links the
plasma membrane to cytoskeletal proteins. Ankyrin 3 was originally
found at the axonal initial segment and nodes of Ranvier of neurons in
the CNS and PNS.
PubMed: 9060470
LocusLink: 288
53BP2
A p53 binding protein that may have a role in p53 related
signal transduction pathways and BCL2, a regulator of programmed cell death.
PubMed: 97035414
LocusLink: 7159
p19INK4d
A cyclin-dependent kinase inhibitor that inhibits CDK4.
This protein is a member of the INK4 family of cyclin-dependent kinase
inhibitors. It prevents the action of of the CDK kinases and controls
cell cycle G1 progression. Also known as CDKN2D.
PubMed: 98013176
LocusLink: 1032
GABP-a/b
GABP (growth-associated binding protein) is a transcription
factor with roles in development, in some cases interacting with members
of the Sp-family of transcription factors. It is a heterodimer.
GABP -a is a member of the ETS-family of transcription
factors, whereas GABP-b consists of ankyrin
repeats.
PubMed: 98128030
PubMed: 10934247
LocusLink 2552
NOTCH3
Third discovered human homologue of Drosophila melanogaster
type I membrane protein notch. This protein interacts with cell-bound
ligands and establishes an intercellular signalling pathway that is key
to neural development in Drosophila. The homologue of this protein
in humans has an undetermined function, but is associated with certain
inherited dementias (OMIM: 125310).
PubMed: 7835890
LocusLink: 4854
Human Disease
Hereditary spherocytosis (OMIM: 182900)
Characterized as one of the most common hereditary hemolytic
anemias among whites of northern European descent. HS is an autosomal
deficiency characterized by spectrin deficient red blood cells resulting
from a mutation in the alpha- and beta-spectrin loci on chromosome 1 and
14 and/or a deletion of an ankyrin 1 copy on chromosome 8 (SPH2).
Hereditary spherocytosis red cells are characterized as spectrin deficient
from both dominant and recessive variants. Human erythrocyte ankyrin
links beta-spectrin to an anion exchange protein AE1. Chromosome
mapping shows that the gene for ankyrin 1 maps to chromosome 8p11.2 and
that one copy was absent from the DNA of two unrelated individuals characterized
with severe HS (Lux et al. (1990) PubMed: 90294909).
The affected red cells in these individuals are also ankyrin-deficient.
Data from Lux et al. suggest that deficiency of ankyrin are responsible
for HS at the SPH2 locus. Coetzer et. al. (1988) also described similar
deficiencies in ankyrin in two patients with severe HS (PubMed: 2961992).
Delaunay also attributes mutations in the genes encoding ANK1 as
a source of HS (PubMed: 12432217).
Submitted by:
Matthew Cambell
Kyle Lorditch
Cell Biology Class
Shippensburg University
11/20/02
Edited by William J. Patrie
12/13/02